
Retinopathy
of Prematurity:
Dr Pablo F. Larrea - Dra Viviana Waisman
Retinopathy of Prematurity (ROP) is a disease that
occurs in premature and low birth weight infants, causing an abnormal
development of the blood vessels of the retina. The retina is the membrane that
covers the interior of the eye globe.
ROP is a vasoproliferative retinopathy. The vascular growth is halted,
causing an abnormal maturation of the blood vessels.
Normal
retinal vascular development:
The retinal vascular development begins at 16 weeks of gestation, from
a mensenchymatic stem in the optical nerve, progressing towards the periphery.
It develops every month as shown in figure 1.

fig 1: vascular development of the retina, by months of
gestation. Note that the nasal side of the eye is completed before the temporal
one.
As the optic nerve is not at
the center of the eye, but towards the nasal zone, the vascular development is
completed on that side at 8 months of gestation. In the temporal side it is
completed between the 9 ½ and 10 months.
Consequently the more premature the baby, the more inmature its
vascular development,
with a big peripheral avascular zone to be covered
by blood vessels.

Fig: 2 schematic anatomy of the
eye, where the retinal blood vessels haven´t reached the ora serrata.
History:
The disease was originally described in 1942 by Terry, with the name
of Retrolental Fibroplasia.
The first epidemic of blind young children took place between 1948 and
52. In the late 50s it was related to oxygen, which was then strictly
controlled, restricting its use in the neonatal care units of the U.S.A.. This caused an abrupt decline in the incidence of
ROP in premature infants, but drastically increased their severe cerebral
damage and deaths. It was estimated that for each blindness prevented,
aproximately 16 children died because of inadequate oxygenation.
The Second Epidemic took place in the 70s, because the technical and
scientific advances allowed increased survival of younger and smaller premature
babies.
In 1980, the
disease was named Retinopathy of Prematurity, leaving the name Fibroplasia Retrolental for the cicatrizales
stages.
As the advances in neonatal care and improvements in technology
increase the survival of very young and
low birth weight premature infants, the number of ROP cases will increase as
well.


Fig 3. Baby in Neonatal Intensive Care Unit
(NICU) Fig: 4 Baby with Oxygen
supplementation
Epidemiology and
Demographics:
Between 1943 and 1951, there
were aproximately 7000 premature children in the U.S.A. blind by ROP. In a
single year (1979) there were 546 blind premature children. And today there are about 500 new blind children by ROP
in the U.S.A. per year.
The survival of a premature baby of 1000 grs. increased considerably
with the technical and medical advances, from 8% in 1950 to 35% in 1980 and 90%
in 1999.
Nowadays
a newborn infant with 25-26 weeks gestation and 750 grs. of birthweight has
a 50% chance of survival.
Risk Factors:
The three most important factors are: birth weight, gestational age
and oxygen.
The lower the weight, the
greater are the possibilities of displaying
some degree of ROP. The relationship is inversely proportional, as seen in this
statistic:
- Birth weight lower than 1000g: ROP incidence 72%
or more.
- Birth weight higher than
1500g: ROP incidence 10% or less.
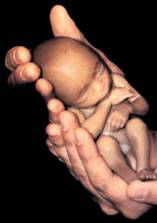
Fig
5: Low weight Baby. Notice he fits inside adult hands.
ROP
Prevalence (according to birth weight) in the U.S.A.
Birth
weight ROP II ROP IV Blindness
< 1000 grs 38 - 54% 22-44% 5-11%
1000
-1500 grs 5-15% 0,7-7% 0,3-1,1%
>
1500 grs 0,6-3% 0,2% 0%
Ophthlmology
1991 Nov;98(11):1628-40 CryoROP Group.
Consequently: a premature baby who weighs 1000 gr. or less at birth has
a 50% chance of having some degree of ROP and a 10% chance of blindness.
There is also an inversely proportional relationship between
gestational age and ROP. The more premature the baby, the higher the risk of
ROP.


Fig 6: baby photo (intrauterine)
For
each additional week the baby stays in the normal uterine environment, there´s
a 27% decline of the probability of reaching a severe stage of ROP. (AAO
Meeting 1996- Dres S Isenberg and Earl Palmer)
Oxygen
supplementation has been associated with this disease for a long time (since
the 50s). But oxygen contribution is an important ally
to save the life and cerebral function of the premature infant, who´s immature
lungs cannot obtain it properly.
Oxygen has an important role in the production of ROP, even tough it´s
not the only factor to be blamed. Too little oxygen (HYPOXIA), as well as too
much (HYPEROXIA), triggers a series of
events that leads to retinopathy.
There are 3 important points to take care of:
Hyperoxia.
Hypoxia/Hyperoxia fluctuations.
Continuous transcutaneous
oxygen monitoring is really important ! !
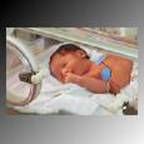

Fig 7: Premature infant in NICU, with oxygen
monitoring connected.
The longer the oxygen therapy is maintained (specially if there´s no
saturation monitoring), and the greater the inspired oxygen fraction, the
higher the odds of developing ROP.
Oxygenation changes cause a perturbation of the vasculogenic
regulators, first by halting the vessels growth, then triggering the events
leading to ROP.
There are two well defined phases:
Obliterative phase:
The
main normal stimulus for the retinal vascular growth is the physiological
Hypoxia of the peripheral retina.
When the infant is receiving oxygen supplementation, (Hyperoxia) this
restrains the normal vascular development by decreasing the release of
endotelial growth factors (VEGF). But the cellular growth and differentiation
in all the retinal layers continues. This “retinal developmental wave” is not
followed by a “vascularization wave”, as this has been stopped by the oxygen
excess.
When the baby´s respiratory function improves, the oxygen support is
suspended, and the following phase begins.
When the baby stops receiving an extra oxygen
contribution, a zone of peripheral retina without blood vessels can´t be
reached by the blood oxygen
This
causes a retinal hypoxia that produces the vasoproliferative phase, by
releasing many substances, specially the
Vascular Endothelial Growth Factor or
VEGF. This process is the same as any hypoxic – vasoproliferative
retinal disease, as diabetic retinopathy or ischemic central retinal vein
occlusion.
The infant´s retina finds out
it´s been in an “Oxygen honey moon” .


fig 8: The posterior zone is vascularized and the
anterior zone is not.
fig 9: The developmental wave of the layers of the
retina, shown in profile, and with a red line the the retinal vessels growth.
Severe hypoxia or respiratory arrest:
increases ROP risk.

Fig 10: Cardiopulmonary resuscitation of a
baby.
Ductus and cardiovascular disease.
Surfactant use: It´s
a well known fact that the exogenous surfactant diminishes the ROP risk.
Light: It´s
been demonstrated that there is no relationship between the increased
ambient illumination (even light theraphy) and the progression of the disease.

Fig
11: Neonatal light theraphy
Carbon dioxide: It´s
vasodilating action would increase the endothelial surface exposed to the toxic effect of oxygen. Some investigators found a link between hypercapnia and ROP
Indometacine:
used for patent ductus treatment, causes vasoconstriction by a change in the
prostaglandins balance. There´s no clear relation between ROP and it´s use.
Transfusions: the
fetal hemoglobine has greater affinity with oxygen than the adult hemoglobine,
so the transfusions with blood or concentrated red cells from adult donors
increase the blood oxygen release.
Vitamin E: It
was used for some years to prevent ROP because of its antioxidant properties,
but an increase in the incidence of necrotizing enterocolitis was noted in
infants with vitamine E supplementation.
In addition, it seems that the ROP severity diminishes but not it´s
incidence, so it´s not widely recommended.
The incidence of ROP in infants less than 1500 g. and/or with less
than 30 weeks gestation at birth has been estimated to be 16 to 56 % depending
on the Neonatal Intensive Care Unit.
ROP appears and develop between the 35 and 45 posconceptional age
weeks.
The first sign of ROP can be detected at 4 weeks of extrauterine life.
Almost all the infants who show
early stages of ROP (stage 1 or 2), will soon complete their vascular
development, with a total resolution of the disease.
The sign of regressed ROP is that the blood vessels continue their
growth anteriorly, surpassing the demarcation line, into the peripheral
avascular zone. This can occur up to 20 weeks after the discovery of the first
signs of the disease.
In a small percentage of these premature infants ROP can evolve to
worse stages, and, specially if not treated, can finish with retinal detachment
and blindness.
As stated before, In infants,
the normal vascular development begins at week 16 of intrauterine life, with a
mesenchymal precursor that grows from the optic nerve, advancing into the peripheral retina, to
reach the ora nasally at 36 to 38 weeks and temporally at 40 to 45 weeks.
The
mesenchymal stem is followed in its migration by spicular cells that are
precursors of the endothelial cells of the retinal blood vessels.

Fig 12:
Intrauterine development of the eye globe.
There
are two theories for the vascular development: vasculogenic theory and
angiogenic theory.
Vasculogenic Theory: endothelial cells are
developed from fusiform cells, as solid chorda that then become hollow to form
blood vessels.
Angiogenic Theory: buds that would form the new blood vessels are developed from
preexisting ones.
These
2 theories are complementary in the normal vascular development of the retina.
The
most important fact is that the vascular growth wave is coordinated with the
wave of retinal layers cellular growth (immediately after it).
In
normal conditions, the limit between the vascular and the avascular retina is
diffuse.
Then a certain toxic agent, possibly the oxygen (it has been shown
that ten hours of oxygen exposure without control can produce a definitive
closure of the normal blood vessels), interrupts the vasculogenic process, and
the surviving vascular channels unite to form a mesenchymal arteriovenous
shunt, and thus remain for days or weeks.
When
the vasculogenesis is resumed, two things can happen:
The arteriovenous shunt cells
get differentiated in normal vascular endothelial cells, and the capillaries
grow into the avascular retina, with complete regression of these early
ROP stages (as occur in more than 90% of the ROP cases).
The other alternative is that
the arteriovenous shunt cells proliferate in an indiferentiated form, as
new vessels towards the surface of the retina. They create fibrovascular
vitreoretinal sheets that pull the retina anteriorly when they contract,
producig a fold, then a traction retinal detachment, and ending as “retrolental
fibroplasia”.
The
factors that determine if the evolution will be one or the other have been investigated, but cannot be controlled.
What is well known is that the most posterior the
disease, meaning a greater extension of
avascular retina, the worse the prognosis. This concept is so important
that it conditions the ROP classification.
Due to the inmature lung function of a premature child, oxygen
supplementation is essential for its neuronal function and life. As previously
explained, this diminishes the release of the retinal blood vessels growth
stimulating factors, producing a “faked normal state”: there is an imbalance between the
surface of retina to be irrigated and the developed blood vessels.
There´s
a peripheral avascular (ischemic) retina, and a posterior avascular retina,
with a whitish thin line between these two zones, neatly separating them. This
is called “Demarcation Line” or ROP
Stage 1.



Fig 13:
Stage 1 ROP.
When the oxygen support is
suspended, the difference between the mature retina energy requirment and the
vascular incomplete oxygen contribution is “discovered” ,
causing the release of vasoproliferative substances (VEGF- Vascular endothelial
growth factor -, MMPs, etc.) and protein production, changing the aspect of the
line that takes on height and width, as a white chord on the retinal surface: “Ridge”
or Stage 2 ROP.



Fig 14: Stage 2 ROP.
If the disease proceeds, new vessels start to grow from the ridge.
Unlike the normal retinal vessels, these are
fragile and are embedded in fibrous tissue. Instead of growing
horizontally in the surface of the retina, they take a vertical direction
towards the Vitreous body. That´s “Extraretinal neovascularization” or ROP
Stage 3.


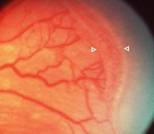
Fig 15: Stage 3 ROP.
At this point progressive vascular incompetence may be noted by
increased tortuosity and dilatation of the retinal vessels, fist the peripheral ones but then the
posterior veins get enlarged and the arteries get tortuous. This is called “Plus
disease”.


Fig 16: Standard photography of Plus disease
The vascular incompetence can be seen as iris
vascular engorgement, vitreous haze or hemorrhage, or pupillary rigidity.
Progression of the disease leads to retinal detachment: “Subtotal
retinal detachment” or ROP Stage 4. Without
involvement of the macula 4a and with macular involvement 4b,
both meaning great vision disturbance.

Fig 17:
Stage 4 ROP.
Finally there´s Stage 5 ROP:
“Total retinal detachment” or Retrolental Fibroplasia with absolute vision
loss of this eye (blindness).
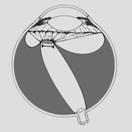


Fig 18:
Stage 5 ROP (Total retinal detachment: scheme)

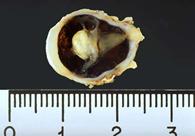
Fig 19: Leukokoria left eye; Stage 5 ROP
(pathology)
In
1984, twenty-three prestigious ophtalmologists of eleven countries met, and
devised a classification system: ICROP, that is still used.
This
classification determined a very important landmark in the understanding of
ROP. The disease was then widely admitted, and this allowed comparison of the
results of treatment techniques by ophthalmologists worldwide.
The
ICROP considered 3 parameters:
The
fundus was divided in three zones:
Zone I or Posterior Pole:
is a circle centred on the disc, that extends twice the distance between
the disc and the center of the macula.
Zone II or Mid-Peripheral
Retina: extends from the Zone I perimeter
to a circular line tangential to the nasal Ora Serrata (which is the peripheral
limit of the neurosensorial retina).
Zone III or Far Peripheral
Retina: It´s the temporal retinal crescent
left between zone II and the temporal Ora Serrata, the last zone to be vascularized.
The
extent is specified as hours of the clock
taken by the disease.


Fig 20: Scheme of ICROP employed to describe
location and extent of ROP.
C
– SEVERITY
The
disease is defined by its severity in Stages:
0
- Vascularization incomplete but with no ROP
1
- Demarcation line
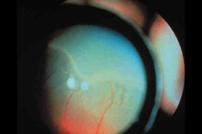

Fig 21:
Stage 1 ROP
2
- Ridge

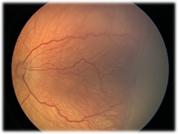
Fig 22: Stage 2 ROP
3
- Ridge with extraretinal fibrovascular
proliferation


Fig 23: Stage 3 ROP.
Any stage can be aggravated by Plus Disease, meaning progressive
vascular incompetence, noted by:
·
Venous engorgement, arterial
tortuosity, specially important if present at the posterior pole.
·
Pupillary rigidity (resistant
to dilatation).
·
Peripheral retinal hemorrhages.
·
Vitreous haze.


Fig 16:
Plus Disease in Posterior Pole
4 - Subtotal Retinal Detachment
4a No macular involvement
4b With macular involvement

Fig 17:
Stage 4 ROP.
5 – Total Retinal Detachment
(several clinical funnel forms: open, open-open, closed-closed, closed-open, open-closed,
closed).

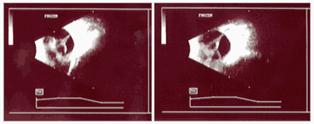
Fig 24:
Leukokoria left eye; Stage 5 ROP
Ultrasonography.
The
disease can be divided in two different phases:
![]()
Stages 1,2, and 3
1.Active Phase: Acute ROP Plus Disease
Rush Disease
There´s a crucial point in the course of ROP that is called “Threshold
Disease”, and is defined as: at least 5 continous or 8 discontinuous clock
hours of Stage 3 ROP in Zone I or II in the presence of Plus Disease. This
definition was used in the CRYO-ROP Study,
a huge multicenter trial that demonstrated that, if treated at this
point, the number of eyes that had an unfavorable outcome was half that of eyes
that were not treated. Thus, this is the
point at which using the right treatment can make bad outcomes much less
frequent.
![]()
2.Inactive
Phase: Cicatricial ROP Stages
4a , 4b, and 5
Regression Sequels
Regression Sequels: most of the cases undergo
spontaneous regression: the retinal vessels cross the disease line, growing
anteriorly to cover all the avascular retina.
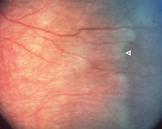
Fig 25:
blood vessel crossing the ROP line (white arrow)
But
regression may not be complete,and the disease may
leave some sequels, like tractions and retinal folds.
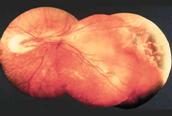
Fig 26:
Nasal retinal fold after transcleral Cryotherapy.

Fig 27:
Macular heterotopia caused by temporal retinal traction.
The
type and severity of ROP retinal sequels depend on what stage it has reached in
the acute phase.
Other
common findings in premature infants are: myopia, astigmatism, strabismus,
subnormal vision, retinal folds, macular heterotropia, tilted disc,
microphtalmos, glaucoma, delayed retinal detachment.
Mode of examination of a premature neonate.

Examination of every premature newborn in the Neonatal Intensive Care
Unit with binocular indirect ophthtalmoscope should be done at 4 WEEKS OF AGE.
The
infant pupils must be dilated. This can be achieved with Fotorretin®, one drop
every 15 minutes, instilled 3 to 4 times.
The
examination is performed with binocular indirect ophthtalmoscope and a
magnifying lens; with topical anesthetic, and a lid speculum (premature type).
The posterior pole must be
evaluated first, then the whole peripheral retina to the Ora Serrata must be
carefully examined, with rotation of the eye and scleral depression.
Temporal big ROP “bays” must be identified,
because they start most retinal folds and macular heterotropia.
Which baby should we examine?
In order to use the same criteria in all the
Neonatal Intensive Care Units in our state, we developed together the “Protocol
for the study of Retinopathy of Prematurity in San Juan”, agreeing to:
Examine every premature infant born with:
- Less than 33 weeks and/or 1500 gr.;
- Less than 35 weeks and/or 2000 gr. that received supplemental
oxygen;
- All those with severe perinatal hyopxia (Apgar 0 to 3 in the first minute and/or 5 at
5 minutes);
- Those with unstable clinical course;
-
Twin whose brother / sister is one of the above.
The examination is done in the
NICU, by a trained ophthalmologist who participate in this work group, with binocular indirect
ophthtalmoscope and scleral depression.
The
initial examination should be done at four weeks of age; and the suggested
follow-up shedule is: Stage 0 to 1: every 2 weeks; Stage 2: every 1 week; Stage
3: every 72 hours.
In
every case The follow-up can be modified up to the observer´s criterion,
depending on the location of the disease and the corrected age of the patient.
After discharge from the NICU the follow up will go on in the ophthalmologist´s
office. This will include examination at one year of age searching for sequels,
including refraction errors, strabismus, etc.
In
the forementioned Protocol was also determined that: “Treatment (Diode laser
photocoagulation delivered with the indirect ophthalmoscope to the entire
anterior avascular retina) will be
indicated according to the following criteria: ROP Stage 3+ in Zone II in 5
continuous or 8 cummulative clock hours ; or ROP any
Stage in Zone I in the presence of plus disease. The gestational age and the
location of the disease are also considered in the treatment decision.
Treatment
can be applied under topical or local anesthesia, with or without sedation; or
under general anesthesia, according to the ophthalmologist´s indication. It is
provided by a neonatologist and/or an anesthesiologist in the NICU or central
operating room.
The
aim of the treatment with Cryo or Laser is the ablation of ischemic retina to
reduce the formation of vasoformative substances and, thus, to produce
regression of neovascularization.


Fig 28: Treatment located on avascular retina.
The
postoperative examination should be performed 5 to 7 days after treatment, looking for non treated (skipped)
areas. The first sign of a favorable effect is the subsiding of the plus
disease within this first few days, then the regression of extraretinal
fibrovascular proliferation in 10 to 20 days. The disease has regressed when there
are normal vessels growing from the vascular retina towards the ora serrata,
crossing the ROP line.
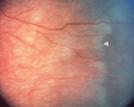
Fig 25: blood vessel crossing the disease line
(white arrow).
If
areas that have not been treated show active disease in the same quadrant,
additional treatment is indicated.
There
are cases that do not respond to treatment, even if photocoagulation (or
cryotherapy) was correctly applied (in time and amount). Some other times the
treatment has to be postponed because the infant is severely ill, and became
less effective. In any of these cases, surgery is the only method left to
restore some useful vision to these eyes.
It´s
used in advanced ROP, stages 4 and 5, trying to obtain, at least, some vision.
There
are 2 types of surgery:
Scleral Buckling:
This surgery is performed without opening the globe, trying to
reattach the retina by lessening the diameter of the globe. A silicone band 2
mm wide is placed encircling the globe, below the extraocular rectus muscles
insertion, to support the area of highest ridge elevation. Additional laser or
cryo is applied to avascular attached retina if active disease is still
present.
We
use it in stage 4b or in cases that did not respond to treatment.
Scleral
buckling must be divided in six months
because it interferes with the normal eye growth, causing significant
myopia.
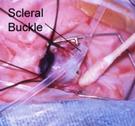
Fig 29:
placing a scleral buckling
Vitreoretinal
Surgery:
It´s reserved to the more advanced stages: 4b if the vitreous traction
is severe and the buckling is not enough to reattach the retina, or 5 with
open-open or open-closed funnel.
Vitreoretinal
surgery in these small eyes has many technical difficulties. Besides, the
children eyes have high probability to become significantly inflammated, many
postoperative complications, and are difficult to control. The results are
quite discouraging, since a high percentage, (60 to 80%), end with no light
perception, and only a few cases have some useful vision.
In cases of closed funnels we prefer not to perform surgery.
Information sources:
ROPARD
Association for Retinopathy of Prematurity and Related Retinal Diseases:
http://www.ropard.org/
Retinopathy of Prematurity :
http://www.konnections.com/eyedoc/ropstart.html
ROP support group
http://www.konnections.com/eyedoc/ropsupp.html
ROP : http://www.growingstrong.org/rophttp://www.growingstrong.org/rop
ROP Links
http://www.growingstrong.org/rop/roplinks.html
ROP - D. Derleth
http://hometown.aol.com/dderleth/ropinfo.html
ROP -Resources at Family Village:
http://www.familyvillage.wisc.edu/lib_rofp.htm
Visual Impairments – Resources at Family Village:
http://www.familyvillage.wisc.edu/lib_blnd.htm
Bibliography:
-Seiberth V, Linderkamp O.: Risk factors in retinopathy of
prematurity. a multivariate statistical analysis.
Ophthalmologica 2000;214(2):131-135
-Brown BA,
Thach AB, Song JC, Marx JL, Kwun RC, Frambach DA: Retinopathy
of prematurity: evaluation of risk factors. Int Ophthalmol 1998;22(5):279-83
-Bassiouny
MR.: Risk factors associated with retinopathy of prematurity: a study from
Oman. J Trop Pediatr 1996 Dec;42(6):355-358
-Olea
Vallejo JL, Corretger Ruhi FJ, Salvat Serra M, Frau Rotger E, Galiana Ferre C,
Fiol Jaume M.: [Risk factors in retinopathy of prematurity]. An Esp Pediatr. 1997
Aug;47(2):172-6. Spanish
- Gellen
B, McIntosh N, McColm JR, Fleck BW: Is
the partial pressure of carbon dioxide in the blood related to the development
of retinopathy of prematurity? Br J Ophthalmol 2001 Sep;85(9):1044-1045
- Cryotherapy
for Retinopathy of Prematurity Cooperative Group: Effect of retinal ablative therapy for
threshold retinopathy of prematurity: results of Goldmann perimetry at the age
of 10 years. Arch Ophthalmol. 2001 Aug;119(8):1200-1.